WHO's latest rabies recommendations and guidance save lives and reduce the cost of treatment
Abstract
Rabies vaccination is a crucial part of rabies post-exposure prophylaxis (PEP), but it tends to consist of long and costly regimens of intramuscular (IM) injections. Most human rabies deaths are caused by delayed access, unaffordability or ineffective delivery of PEP. Reducing these barriers is crucial to ensure that this incurable yet preventable disease does not cost lives. In 2022, WHO published new guidance towards the introduction or expansion of rabies vaccination into national immunization programmes to systematically drive down human rabies deaths effectively and cost-efficiently. Such guidance grounds on the latest scientific recommendation provided by WHO’s Strategic Advisory Group of Experts in 2018. WHO recommends a shortened 1-week rabies vaccination schedule, with visits on days 0, 3 and 7. On each visit, a 2-site intradermal (ID) injection (using only 0.1 ml of vaccine in each site) is administered. ID administration allows for vials to be shared among several patients within a 6-8 hours timeline. Compared to IM administration, ID is cost- and dose-sparing, even in low-throughput clinics. Additionally, this regimen requires only 3 visits to the healthcare facility, improving patient compliance. However, the uptake of this shortened ID regimen remains limited. It should now be a matter of urgency for Health Ministries in rabies-endemic settings to adopt the WHO-recommended shortened ID vaccination schedule and ensure appropriate medical training to improve PEP delivery. This will enable countries to improve PEP delivery and allow underserved populations to access affordable, life-saving rabies vaccines.
Keywords
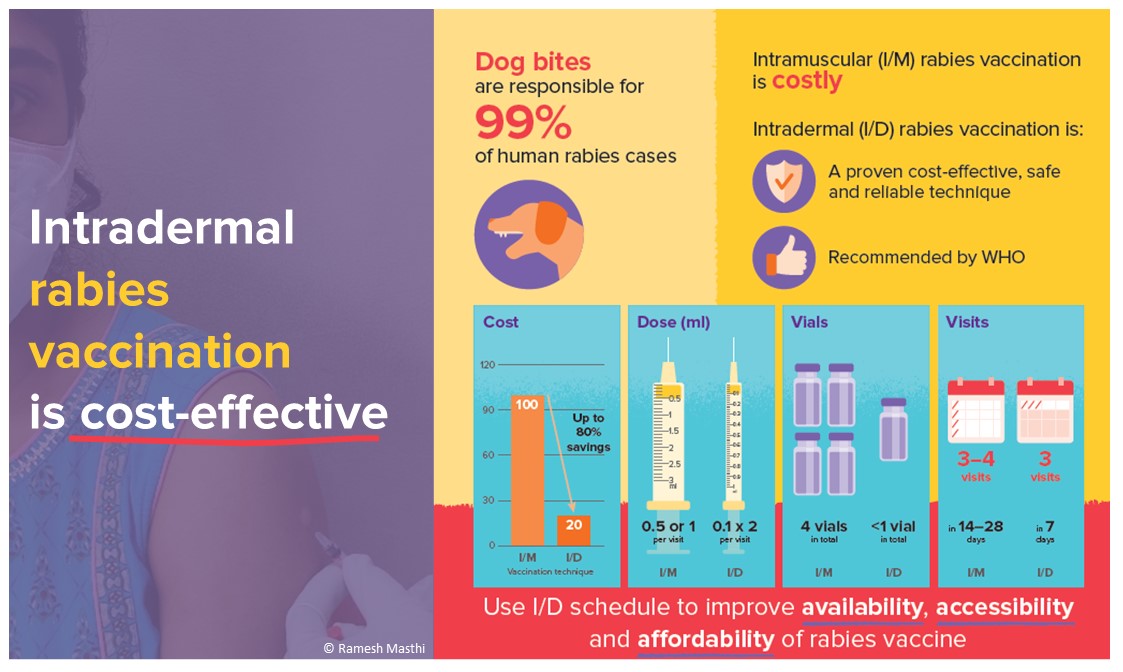
Rabies is a viral zoonotic disease that remains a significant - and deadly - public health problem in many parts of the world. Rabies infection causes an estimated 59,000 human deaths each year, mainly in underserved populations in Asia and Africa, and 40% of the victims are children under 15 years of age. In rabies-endemic areas, contact with domestic dogs through bites or scratches is responsible for 99% of the cases[1].
In 2018, the World Health Organization (WHO), the Food and Agriculture Organization of the United Nations, the World Organisation for Animal Health and the Global Alliance for Rabies Control set the global target of achieving zero human deaths from dog-transmitted rabies by 2030 (“Zero by 30”)[2]. As rabies is almost inevitably fatal as soon as symptoms appear, its prevention, both in humans and dogs, is key to reaching this goal. Prevention requires providing post-exposure prophylaxis (PEP) to exposed individuals, mass vaccinating susceptible dog populations to control the disease at its source, and increasing community awareness and engagement in rabies control.
PEP consists of thorough wound washing for 15 minutes with copious amounts of water and soap, a series of rabies vaccine injections, and, if indicated, the administration of rabies immunoglobulin or monoclonal antibodies. Since Louis Pasteur’s first efforts in 1885, rabies vaccines have been greatly improved and modern vaccines are highly immunogenic and well-tolerated. Most vaccines were initially developed for intramuscular (IM) administration; therefore, the standard vial size corresponds with a single dose of IM vaccine (either 0.5 or 1 ml, depending on the manufacturer). Depending on the chosen IM PEP regimen, at least 4 doses of vaccine are needed. Though there are safe and effective rabies biologicals, especially in low-income settings, rabies continues to kill those who cannot access healthcare timely and cannot receive effective PEP, due to its unavailability or unaffordability. Expanding rabies PEP is a critical step in saving lives and ending the burden of rabies. In 2018, GAVI, The Vaccine Alliance, decided to add rabies to their vaccine investment strategy, paving the way for greater access to PEP worldwide[3].
As part of its commitment to the Zero by 30 goal, WHO works to provide rabies immunization guidance intended to reduce barriers to the access to and delivery of PEP. In 2022, WHO published a new guide for the introduction or expansion of rabies vaccination into national immunization programmes to systematically drive down human rabies deaths effectively and cost-efficiently[4].
For exposed individuals who have not been previously immunized, WHO recommends a 1-week vaccination schedule on days 0, 3 and 7. On each visit, the rabies vaccine is administered through 2-site intradermal (ID) injections of 0.1 ml of vaccine each, preferably using an insulin syringe[5,6]. This shortened ID regimen is as efficacious as other established regimens since the antigen-presenting cells in the skin are more effective than the same cells in the muscle, thus being able to trigger a high-immune response[7,8]. Moreover, one ID vaccination dose only requires 0.1 ml of vaccine per site allowing for one vial to be shared among patients (subject to hygienically using a new syringe each time and properly maintaining the cold chain). Once opened, vials need to be discarded after 6-8 hours. Before disposing of the open vial, it is recommended to use the remaining vaccine for PrEP in animal and human health professionals at occupational risk, or relatives or accompanying persons of exposed patients.
Considering that less vaccine is needed per injection and fewer visits are required due to the shortened vaccination schedule, ID vaccination has been proven cost-effective even in low-throughput clinics. As throughput increases, ID regimens become increasingly cost-effective, saving up to 85% of the available vaccine[9]. This increases the number of available vaccines, preventing shortages due to supply issues or financial constraints in the healthcare system. Following the recommendations for ID vaccination would hence not only avert additional deaths but also be highly cost-effective[10]. Additionally, it would make vaccination more affordable for patients, which is one of the main obstacles to timely PEP-seeking in countries where the healthcare system does not provide PEP for free[11]. Increasing the affordability of PEP not only saves lives but is also in line with the United Nations’ Sustainable Development Goals. In particular, it ensures good health (Goal 3), reduces inequalities (Goal 10), and when vaccination is not covered by government policies, it decreases out-of-pocket spending in communities that are often already stricken by poverty (Goal 1).
However, despite the described advantages, most countries have not yet adopted the shortened ID vaccination schedule and the information leaflets of most rabies vaccines have not been updated to include the 3-visit ID regimen. Switching to ID vaccination would offer countries an important chance to tackle PEP-related challenges in times of crisis, for example, when vaccine supply or adequate logistics are insufficient, such as during acute outbreaks with many dog rabies cases or events like the COVID-19 pandemic when health systems are under extreme stress[11], but also in normal times. Seeking PEP not only comes with the cost of the treatment itself but further includes the cost of travelling to the healthcare facility and the loss of time and income. Patients living in rural areas, who have to travel long distances to reach a healthcare facility, may easily feel discouraged by long vaccination schedules. In underserved areas, public transportation is often the only option available, but the length of such travel, coupled with reduced financial means, may prevent people from starting or completing PEP. The shortened vaccination regimen increases the likelihood of patients receiving all the doses of vaccine they need.
Misinformation about rabies vaccination is an additional obstacle and can create vaccine hesitancy, refusal and scepticism. Healthcare workers need to be trained to communicate with the public about safety and concerns[4]. A shortened vaccination schedule with fewer visits decreases the patient load in high-throughput clinics and allows healthcare workers to treat and inform their patients with less stress and more time.
Adopting the recommended ID vaccination schedule further assists countries in their efforts to include rabies in routine vaccination programmes because, especially in the initial phase of any programme, vaccine demand can increase due to augmented awareness. Using dose-sparing ID regimens would assist in ensuring the vaccine supply and preventing shortages. Countries could then focus on stable vaccine procurement, especially of WHO pre-qualified rabies vaccines that make up only 14% of the total share[12]. The upcoming Gavi, the Vaccine Alliance’s support focused on ID vaccination is expected to attract additional attention and accelerate the shift to ID administration[13].
In combination with mass dog vaccination, investment in human PEP using the WHO’s recommended 3-visit ID vaccination schedule will drive progress towards the stated aim of zero human deaths from dog-mediated rabies by 2030. It should now be a matter of urgency for Health Ministries in rabies-endemic settings to adopt this beneficial regimen and ensure appropriate medical training and healthcare service delivery to improve PEP, enabling underserved populations to access affordable, life-saving rabies vaccines. The “Guide to introducing human rabies vaccine into national immunization programmes” supports Health Ministries in their policy discussions and 6-step operational planning towards this goal[4].
DECLARATIONS
Authors’ contributionsConceptualization and writing this comment: Bote K, Nadal D, Abela B
Conceptualization, writing - original draft: Bote K
Conceptualization, writing - review & editing: Nadal D
Conceptualization, writing - review & editing: Abela B
All authors read and approved the final manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 2015;9:e0003709.
2. World Health Organization, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health. Zero by 30: the global strategic plan to end human deaths from dog-mediated rabies by 2030. Available from: https://apps.who.int/iris/handle/10665/272756 [Last accessed on 20 Mar 2023].
3. Gavi, The Vaccine Alliance. Vaccine investment strategy. Available from: https://www.gavi.org/our-alliance/strategy/vaccine-investment-strategy [Last accessed on 20 Mar 2023].
4. World Health Organization. Guide to introducing human rabies vaccine into national immunization programmes. Available from: https://apps.who.int/iris/handle/10665/360978 [Last accessed on 20 Mar 2023].
5. World Health Organization. Rabies vaccines: WHO position paper - April 2018 - vaccins antirabiques: note de synthèse de l’OMS - avril. Available from: https://apps.who.int/iris/handle/10665/272372 [Last accessed on 20 Mar 2023].
6. World Health Organization. WHO expert consultation on rabies: third report. Available from: https://apps.who.int/iris/handle/10665/272364 [Last accessed on 20 Mar 2023].
7. Cantaert T, Borand L, Kergoat L, et al. A 1-week intradermal dose-sparing regimen for rabies post-exposure prophylaxis (RESIST-2): an observational cohort study. Lancet Infect Dis 2019;19:1355-62.
8. Kessels J, Tarantola A, Salahuddin N, Blumberg L, Knopf L. Rabies post-exposure prophylaxis: a systematic review on abridged vaccination schedules and the effect of changing administration routes during a single course. Vaccine 2019;37 Suppl 1:A107-17.
9. World Health Organization. Strategic advisory group of experts on immunization (SAGE) - October 2017. Available from: https://www.who.int/news-room/events/detail/2017/10/17/default-calendar/strategic-advisory-group-of-experts-on-immunization-(sage)---october-2017 [Last accessed on 20 Mar 2023].
10. Hampson K, Abela-Ridder B, Bharti O, et al. Modelling to inform prophylaxis regimens to prevent human rabies. Vaccine 2019;37 Suppl 1:A166-73.
11. Nadal D, Abela-ridder B, Beeching S, et al. The impact of the first year of the COVID-19 Pandemic on canine rabies control efforts: a mixed-methods study of observations about the present and lessons for the future. Front Trop Dis 2022;3:866811.
12. World Health Organization. Global market study: human rabies vaccines. Available from: https://www.who.int/publications/m/item/who-human-rabies-vaccines-global-market-study-december-2020 [Last accessed on 20 Mar 2023].
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Bote K, Nadal D, Abela B. WHO's latest rabies recommendations and guidance save lives and reduce the cost of treatment. One Health Implement Res 2023;3:11-5. http://dx.doi.org/10.20517/ohir.2022.46
AMA Style
Bote K, Nadal D, Abela B. WHO's latest rabies recommendations and guidance save lives and reduce the cost of treatment. One Health & Implementation Research. 2023; 3(1): 11-5. http://dx.doi.org/10.20517/ohir.2022.46
Chicago/Turabian Style
Bote, Katrin, Deborah Nadal, Bernadette Abela. 2023. "WHO's latest rabies recommendations and guidance save lives and reduce the cost of treatment" One Health & Implementation Research. 3, no.1: 11-5. http://dx.doi.org/10.20517/ohir.2022.46
ACS Style
Bote, K.; Nadal D.; Abela B. WHO's latest rabies recommendations and guidance save lives and reduce the cost of treatment. One. Health Implement. Res. 2023, 3, 11-5. http://dx.doi.org/10.20517/ohir.2022.46
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 15 clicks
Cite This Article 15 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.