Ecoepidemiology of dengue in Brazil: from the virus to the environment
Abstract
Dengue is an infectious disease caused by the dengue virus. In Brazil, the main vector is the mosquito Aedes aegypti (L.); however, Aedes albopictus (Skuse) can also transmit this pathogen. According to the WHO, more than 125 countries are endemic for dengue, and an estimated 50-100 million infections occur annually. In Brazil, the Northeast region is one with high incidence rates and records of successive epidemics. Dengue has been circulating in Brazil for over 30 years, due to the fact that there are areas that offer favorable environmental conditions, as well as municipalities with low socioeconomic conditions and frequent water crises. In addition, Brazil is a tourist hub with an intense flow of visitors, keeping the doors open for the entry and circulation of vector-borne diseases. The role of epidemiology is to analyze the distribution of diseases and their determinants in space and time and to unveil the social inequalities that influence the health-disease process. Thus, a review study that describes the occurrence of Dengue may provide a perspective of future areas of greater risk for dengue epidemics.
Keywords
INTRODUCTION
Approximately 60% of the infectious diseases that affect humans have an animal origin. Most arboviruses are maintained in complex sylvatic cycles that involve mosquitoes and vertebrates that live in forests and are considered reservoirs, such as birds, small mammals, and non-human primates. The amplification of these wild cycles occurs accidentally, causing disease in humans, and consequently, viral adaptation can give rise to urban cycles and epidemics[1,2]. The dengue virus has always presented itself as a serious zoonosis, and this highlights the need to adopt a One Health approach, as a number of reports suggest other animals may play a role in both the sylvatic and urban endemic cycles as potential secondary hosts. Moreover, with increasing deforestation due to globalization and urbanization, animal and human populations are increasingly staying closer together with a higher frequency of potential contacts[3]. It seems plausible that some animals in an urban setting may play an indirect role in dengue ecology, possibly even with low titers of viral load. It allows us to include interacting factors such as the environment, land management, and social and climatic factors that influence the transmission patterns of the disease[3,4].
Dengue is an infectious disease whose etiological agent is the dengue virus (DENV). It is an arbovirus of the Flaviviridae family and Flavivirus genus, which antigenically comprises four distinct serotypes: DENV-I, DENV-II, DENV-III, and DENV-IV[5]. Infection with one of these serotypes confers permanent immunity to it and is believed to provide protection for 1-3 years against a different serotype[6-8].
The virus structure is characterized by an approximately smooth surface with a diameter of 50 Å and a dense core surrounded by a lipid bilayer. The genome is composed of about 10,700 nucleotides, and there are three proteins important for its structure: the capsid protein (C protein) composed of 100 AA; the membrane protein (M protein) formed by 75 AA; and the envelope protein (E protein) composed of
As with other arboviruses, DENV has its genetic code in the form of single-stranded RNA, reflecting high genetic plasticity, a fundamental attribute to complete the transmission cycles that involve replication in mosquitoes and vertebrate hosts[8].
Virus replication in humans starts when the viral envelope proteins (E protein) interact with receptors on the target cells and, through endocytosis, enter the cell in the form of an endosome. The virus then fuses to the membrane of the endosome and the viral RNA is released into the cytoplasm of the host cell, initiating replication. Using the ribosomes of the rough endoplasmic reticulum (ER), the virus translates its RNA, producing a polyprotein with approximately 3400 AA that is cleaved into three structural proteins (C, M, and E) and seven other non-structural proteins[10,11].
The newly synthesized viral RNA is packaged by C proteins to form the nucleocapsid, which is taken to the lumen of the ER and surrounded by the M and E proteins, producing the viral envelope and the outer membrane. Then, the immature viruses pass through the Golgi complex, where they are converted into their infectious form and released to infect other cells[11].
GENERAL ASPECTS OF DENGUE
The main vector of dengue in Brazil is the Aedes aegypti (L.) mosquito; however, the Aedes albopictus (Skuse) mosquito can also act as a vector[12-15]. Both species are dipterous insects of the family Culicidae, belonging to the subfamily Culicinae. In its adult form, Aedes aegypti has dark or nearly black coloration, with white stripes distributed throughout the body and legs; clypeus with two tufts of silver-white scales; and shields also adorned with silver-white scales that form a lyre-shaped design. Ae. albopictus has a morphology similar to Ae. aegypti; however, in its adult form, the shield has only one stripe formed by silver-white scales[16].
Species of the genus Aedes, similar to other Culicidae, are holometabulous insects, and their life cycle comprises four stages: egg, larva (subdivided into four stages: L1, L2, L3, and L4), pupa, and adult[16]. Mosquitoes of the tribe Aedini, when adults, feed on nectar and sugar solutions; however, after copulation, the females require blood as a source of protein needed for the maturation of eggs. Oviposition usually occurs 2-3 days after the bloodmeal[16,17].
To perform oviposition, the female seeks breeding sites that contain standing water. At first, it was thought that the aquatic forms of Ae. aegypti developed exclusively in artificial containers such as tires, cans, plant pots, water tanks, and uncovered cisterns, because they serve as deposits for fresh, still water with a low concentration of organic matter. However, adapting to manmade conditions, it is known that it is possible to find larvae even in rudimentary pits[16,18].
Similarly, it was believed that Ae. albopictus had a wilder behavior, often laying its eggs in natural breeding sites, such as bamboo internodes and tree cavities. However, there are reports of its larvae being found in artificial deposits, and, due to its eclectic character in the choice of breeding sites, there is a significant adaptation of the species to the urban environment[16,19].
On finding a breeding site, the female lays its eggs on the side walls of the reservoir, above the water line; shortly after contact with the water, the eggs hatch into larvae. At this stage, the species is very active, and the larvae in the four stages feed on microorganisms and organic debris present in the water, gaining energy for the next stage. The passage from one stage to the next occurs through the process of ecdysis, where the exoskeleton is detached[17].
When the mosquito reaches the pupal stage, it does not feed and only breathes on the water surface, spending much of the time immobile. On average, after two days in the pupal stage, the adult mosquito hatches. The entire life cycle can last from seven to ten days, depending on the nutrients available and the temperature of the environment, and may last for more or fewer days[17].
The most common way for mosquitoes to become infected with DENV is through blood-feeding on infected hosts; however, vertical transmission is also possible, which occurs when an infected female transmits the virus transovarially to its offspring[20,21].
After the female mosquito bites an infected human, DENV starts replicating in the midgut of the mosquito. It then spreads through the hemolymph to other organs until it infects the salivary gland. It takes 8-12 days for the mosquito to become infective, corresponding to the extrinsic incubation period[22].
The most viable way to reduce the incidence of dengue, and other arboviruses, is using integrated vector control. For this, the forms of control are basically divided into three categories: chemical, biological, and mechanical. Chemical control was the first form of mosquito control used in public health and consisted of the application of chemicals for adults (adulticides) and larvae (larvicides)[23,24]. It is not currently the most recommended measure, given the possibility of selecting strains of mosquitoes that are increasingly resistant to the products as well as the negative impacts on the environment and human population[23,24].
For biological control, predators or pathogens can be used to reduce the vector population, most often in the larval stage. Among the organisms used are fish, aquatic invertebrates, mosquitoes that disperse larvicides or microorganisms (such as the bacterium Bacillus thuringiensis israelensis, which has a potent larvicidal action), and Wolbachia[24,25].
Wolbachia can be considered a recent control approach, especially in Brazil, but it proposes to occur naturally and is self-sustaining in the long term. This is a species of bacteria naturally found in about 60% of insects and, when present in Aedes aegypti is able to halve the life span of an adult mosquito and generate sterile offspring. This species may also be able to eliminate arbovirus transmission by the mosquito through competition for amino acids between the host mosquito and the virus[20].
Mechanical control is based on practices conducive to eliminating the vector and its breeding sites or that reduce the contact of the mosquito with human beings. Activities such as drainage of reservoirs, use of screens on doors and windows, and sealing of water storage tanks are considered mechanical control[24,26,27].
To integrate the control methods, an approach known as eco-bio-social has been increasingly used, with education and social mobilization as its main axes of execution, seeking to extinguish the chemical control of actions to combat dengue. This approach uses health professionals, such as endemic agents and community health agents, as well as voluntary residents of neighborhoods affected by the disease[28]. This is a challenging and successful approach in the long term, but it requires the involvement of the community and various sectors such as sanitation, cleaning, culture, public security, and public agencies. It offers a promising alternative to vector control measures[28,29].
Humans are the only vertebrate host susceptible to developing the clinical forms of dengue and may present from inapparent infections to a series of well-defined symptoms[5,30].
When symptomatic, dengue is considered a systemic and dynamic disease and can be classified into three clinical phases. The first is the febrile phase, whose manifestations are: sudden high fever, headache, muscle pain, myalgia, arthralgia, photophobia, retro-orbital pain, rash, and itchy skin. After this phase, most patients recover with the help of appropriate treatment[31-33].
The second phase is the critical one, and it may be present in some symptomatic patients. It is subdivided into dengue with alarm signs and severe dengue. Dengue with alarm signs may occur when there is an increase in vascular permeability, causing alarming signs such as the onset of severe dengue. Its evolution may cause shock due to plasma extravasation. The main signs are intense abdominal pain, constant vomiting, ascites, postural hypotension, and bleeding of mucous membranes[31].
Severe dengue may manifest with plasma leakage, leading to hypovolemic shock (loss of large volume of plasma), severe hemorrhage, or even dysfunction of several organs such as the heart, lungs, kidneys, liver, and the central nervous system. These manifestations can lead the patient to death within 48 h[31,33].
Patients who survive dengue with alarm signs or severe dengue reach the recovery phase, with gradual reabsorption of all lost plasma content, resulting in a sensible clinical improvement. However, it is still a delicate phase, susceptible to complications related to hyperhydration or bacterial infections, which may contribute to death[5].
The evolution to severe forms is still not completely understood. However, there are three main theories that seek to explain it: Rosen’s theory, Halstead’s theory, and the integral theory of multicausality. Rosen’s theory associates the occurrence of severe dengue with the virulence of the infecting strain[27,34]. The theory defended by Halstead relates cases of severe dengue to sequential infections in a human by different serotypes. Immunological amplification in secondary infection causes an increase in viremia, stimulating the production of more cytokines and proteases that trigger predictive factors for plasma leakage[27,35,36].
In the integral theory of multicausality, it is proposed that there are some risk factors that promote conditions for cases of severe dengue. The factors include age, pregnancy, sex, pre-existence of chronic diseases, circulating serotypes and virulence of strains, and sequence of infections, among others[27,37].
In addition to the classic symptoms related to severe dengue, cases presenting atypical clinical manifestations have been increasingly identified. When the virus directly reaches the central nervous system or the infection affects the peripheral nerves, it results in neurological and/or neuroimmunological manifestations. In recent studies, Guillain-Barré syndrome (GBS) has been shown to be strongly associated with infections caused by DENV[38,39].
GBS is an acute idiopathic demyelinating polyradiculoneuropathic disease, which results from multifocal infiltration by mononuclear inflammatory cells into the myelin sheath or autoimmune antibody-mediated destruction of the myelin sheath and is the most common cause of acute generalized paralysis in the world[39].
According to Fragoso et al.[38], GBS is a rare condition, with few cases described in the literature, but it is still a very underreported manifestation, even in dengue-endemic areas.
Symptomatic humans are key elements in the continuity of the dengue virus transmission cycle, but it is known that its spread can be driven mainly by asymptomatic or clinically inapparent people[40,41]. According to Duong et al.[41], asymptomatic and pre-symptomatic patients infect more mosquitoes than symptomatic patients at any viremia level. This is due to the human immune response, which, by stimulating high levels of cytosine during the disease (which occurs with symptomatic patients), influences the reduction of the transmissibility of the virus to mosquitoes[41]. Thus, it is assumed that the mechanism of virus transmission from human to mosquito can be considered as “silent” because it occurs mostly before the onset of symptoms (reaching its peak on the second day) or in the absence of clinical signs, reducing transmissibility as the days pass or with the increase in IgG and IgM titers[41].
HISTORY AND EPIDEMIOLOGY OF DENGUE
Dengue has a complex and long history of interaction with man, probably beginning in the Asian continent as early as the third century. In China, there are records of a disease with a clinical description very similar to dengue, and this was repeated in the 7th and 10th centuries, during the Tang and Sung dynasties, respectively. Already at that time, this disease was associated with the presence of mosquitoes and water and became known as “water poison”[8].
After half a century, suspected cases of dengue fever were reported in the French Antilles and Panama, beginning its spread in the Americas[42]. Further records of diseases similar to dengue were noted in several places, suggesting the possibility of a pandemic. This rapid spread of the disease coincided with the increase in export and import trade, carried out by sailing ships during the period of the great voyages[8].
The transmission of DENV has accompanied the spread of its main mosquito vector, Ae. aegypti, and has probably been accelerated by disorderly urbanization and globalization[43]. Due to the infestation of Ae. aegypti in the Americas and the occurrence of yellow fever, there was a mosquito eradication program, which eliminated the mosquito from 23 countries[44].
For almost thirty years, there was an “epidemiological silence” with few reported cases of dengue fever. However, in 1970, the control program was interrupted, and a new wave of infestation began. Approximately 20 years later, the mosquito was already present in almost the entire geographical extension of the Americas, causing outbreaks and epidemics by different serotypes[44].
It is possible that the exponential growth of the urban population in Latin America and the Caribbean has also contributed to the hyperendemicity of DENV in the Americas. In addition to the lack of entomological surveillance, uncontrolled urbanization, poor sanitation in urban centers, and the rapid expansion of international and domestic travel have passively favored the dispersal of the mosquito[43].
According to the WHO, more than 125 countries are endemic for dengue, and an estimated 50-100 million infections occur annually, with two-fifths of the world’s population living in areas at risk. From 1990 to 2015, 262 epidemics were recorded worldwide, all occurring in tropical and subtropical regions. Countries with the highest number of epidemics include India with 58, China with 38, and Brazil with 24. Europe was the continent least affected by dengue epidemics, recording only four in France and two in Portugal[45].
Previously, countries in Southeast Asia and the Western Pacific region were the most affected. However, approximately 40 years ago, there was an excessive increase in the number of cases in the Americas. During the 1980s, 1,033,417 cases were reported, 2,725,405 in the 1990s, and, from 2000 to 2007, there were 4,759,007 cases[44].
Between 2010 and 2014, there was a major expansion of DENV-4 to South American countries such as Brazil, Paraguay, and Argentina. There was also an expansion of DENV-2 and DENV-3 to the south of the United States. Along with the dispersion of viruses, there was an increase in the number of cases in the continent, with a proportional constancy in deaths from dengue[46].
The years 2015 and 2016 are highlighted by the high number of cases; however, it corresponds to the period of expansion of the Zika and Chikungunya viruses. Due to the similarity between the symptoms of these arboviruses, clinical diagnosis is difficult, directly influencing the notifications[47] [Table 1].
Dengue in the Americas (2010-2019)
| Year | Dengue and DHF cases | Dengue and DHF deaths (%) |
| 2010 | 1,648,403 | 1188 (1.95%) |
| 2011 | 1,073,051 | 758 (0.07%) |
| 2012 | 1,164,245 | 807 (0.06%) |
| 2013 | 2,384,359 | 1403 (0.05%) |
| 2014 | 1,184,840 | 683 (0.05%) |
| 2015 | 2,415,693 | 1355 (0.05%) |
| 2016 | 2,174,827 | 915 (0.04%) |
| 2017 | 580,640 | 317 (0.05%) |
| 2018 | 561,231 | 336 (0.05%) |
| 2019 | 3,140,872 | 1535 (0.04) |
| 2020 | 2,326,115 | 1027 (0.04%) |
During this period, Brazil was the country in the Americas region that recorded the highest number of cases, with the co-circulation of the four serotypes[46]. In 2016, Brazil contributed to the epidemic in the Americas with more than 1.5 million cases and 1032 deaths[47] [Table 2].
The number of dengue cases in the Americas and Brazil (2010-2019)
| Year | Dengue and DHF cases | Cases in Brazil (%) |
| 2010 | 1,648,403 | 1,004,392 (60.30%) |
| 2011 | 1,073,051 | 764,032 (69.80%) |
| 2012 | 1,164,245 | 565,510 (50.45%) |
| 2013 | 2,384,359 | 1,468,873 (61.50%) |
| 2014 | 1,184,840 | 659,051 (72.50%) |
| 2015 | 2,415,693 | 1,649,008 (68.26%) |
| 2016 | 2,174,827 | 1,500,535 (69.00%) |
| 2017 | 580,640 | 252,054 (43.40%) |
| 2018 | 561,231 | 265,934 (47.38%) |
| 2019 | 3,140,872 | 2,226,914 (70.00%) |
| 2020 | 2,326,115 | 1,467,142 (63.00%) |
DENGUE FEVER IN BRAZIL: THE LANDING THAT RESULTED IN EPIDEMICS
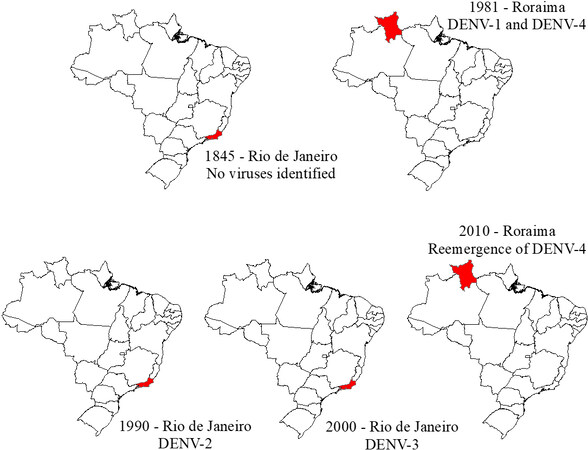
The history of dengue in Brazil began in 1845 when the first epidemic was recorded in the state of Rio de Janeiro. Other epidemics were reported during 1851-1853 and 1916-1923, but the first laboratory-confirmed epidemic occurred in Boa Vista in 1981[13,48].
Five years later, the virus reappeared in Nova Iguaçu (RJ) and since then has spread to other cities in the state of Rio de Janeiro. At that time, a sero-epidemiological survey was conducted, and it was recorded that more than one million people were infected by the virus. At that time, serotype DENV-2 was introduced, and not only the municipalities of Rio de Janeiro state were affected by the numerous cases but other municipalities distributed in the Southeast and Northeast regions of Brazil as well[13]. Between 1986 and 1987, municipalities in these two regions built an epidemiological scenario similar to that of Rio de Janeiro; for example, Alagoas, Ceará, Pernambuco, Bahia, and Minas Gerais counted 43,000 confirmed cases[13]. After these epidemics, dengue spread throughout the country, and 80 years after the first record of the disease in Brazil, it was already present in more than 50% of municipalities[15].
In 2000, the DENV-3 serotype began circulating in the state of Rio de Janeiro[48]. DENV-3 became the most prevalent in the country after its introduction, at the same time that serotypes DENV-1 and DENV-2 also spread throughout the country, increasing the number of fatal dengue cases. This points positively to the hypothesis that a previous infection by a dengue serotype can probably generate a more lethal scenario when a new serotype is introduced[44,49].
In 2010, Brazil again experienced new epidemics, but this time 21 of the 27 states already suffered from the co-circulation of the fourth serotype, including Roraima, which, after 28 years, was again infected with serotype DENV-4[50,51] [Figure 1].
Figure 1. Timeline of introduction of dengue serotypes in Brazil. Source: Modified from Fares et al.[48].
In 2014, 591,080 probable cases of dengue were reported in the country until the 53rd epidemiological week (28 December 2014 to 3 January 2015). The Southeast region had the highest number of probable cases, with 52.8% in relation to the whole country. However, the Mid-West region had the highest incidence, with a coefficient of 754.4/100,000 inhabitants. Compared to 2013, there was a 59.3% reduction in cases in the country. During that year, the most circulating serotype was DENV-1 (82%), and there was a total of 410 confirmed deaths[52].
In 2015, the number of probable cases was three times that of the previous year. The Southeast remained the region with the highest number of notifications, totaling 62.2% in relation to the whole country, although the Mid-West region again recorded the highest incidence coefficient in the country. The Northeast region recorded the second-highest number of probable cases in the country, with a total of 311,519 cases (18.9%). In total, 863 deaths from dengue were confirmed, and serotype DENV-1 continued to be the most circulating[53].
In 2016, the incidence was 733.4 cases/100,000 inhabitants. Again, the Southeast and Northeast regions recorded the most probable cases of dengue, with 57.2% and 21.6 % of the total cases in the country, respectively. The Mid-West region continued to record the highest incidence coefficient[54].
In comparison with 2016, there was a reduction in cases in 2017, registering an incidence of 122.3 cases/100,000 inhabitants. In that year, the Northeast region presented the highest number of probable cases in relation to the country’s total (86,386 cases; 34.3%), with the second highest incidence[55].
In 2018, until the 52nd epidemiological week, a smaller number of probable cases of dengue were recorded in the country compared to 2017, with an incidence of 118.7 cases/100,000 inhabitants. Already in this period, the Centre-West region was the one that recorded the most cases, followed by the Northeast region[55]. In 2019, an increase in the number of dengue cases in Brazil was observed mainly in the Mid-west and Southeast regions. The Northeast had the third highest incidence in the country, tripling the incidence compared to the previous year[56] [Table 3].
Dengue incidence per 100,000 inhabitants, by Brazilian’s regions from 2014 to 2019
| Brazilian region | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
| North | 287.0 | 179.8 | 224.7 | 128.0 | 100.6 | 195.8 | 119.5 |
| Northeast | 160.5 | 578.5 | 573.3 | 151.8 | 118.5 | 376.7 | 263.8 |
| Southeast | 366.9 | 1221.4 | 1001.2 | 69.0 | 86.0 | 1159.4 | 379.4 |
| South | 83.5 | 176.8 | 250.4 | 15.9 | 9.0 | 165.2 | 940 |
| Mid-West | 754.4 | 1496.6 | 1322.0 | 502.7 | 635.9 | 1349.1 | 1212.1 |
The Northeast is one of the regions with high incidence coefficients and with records of successive epidemics. As of 2016, states such as Rio Grande do Norte, Ceará, and Bahia had incidence coefficients above 1500 cases/100,000 inhabitants. In addition, Pernambuco and Alagoas recorded the highest proportion of deaths respective to severe cases or with alarm signs[54].
An important area in Brazil for dengue epidemics is the state of Ceará, located in Brazil’s Northeast. The first record of dengue in Ceará was in 1986, four years after the proven circulation of the virus in the country. The vector Ae. aegypti had already spread definitively in the state since 1984; then, the arrival of tourists from Rio de Janeiro infected with the virus started the chain of transmission and the spread of the disease in the region[57]. In that year, the first epidemic occurred in the state, and it lasted until November 1987, with a total of 30,000 cases caused only by serotype DENV-1[58,59].
By 1993, more than 50,000 cases were reported, and, in 1994, the second epidemic occurred in Ceará. At that time, the first cases of severe dengue fever (then classified as dengue hemorrhagic fever), related to the circulation of DENV-2, were confirmed. The incidence coefficient was 732.31/100,000 inhabitants, and, for the first time, the lethality coefficient reached 48%[57,59].
In that year, Ceará had 84% of the dengue notifications in Brazil, and cases were still underreported, since about 25% of the respective dengue records were confused with rubella. At that time, dengue was not a compulsorily notifiable disease and physicians were not in the habit of spontaneous notification in Brazil[60,61].
The cases occurred more frequently in the months of March to August, peaking in June with 16,608 cases, mostly residents of Fortaleza and Caucaia (a city bordering Fortaleza). The patients who died were autopsied, and the main changes found were gastrointestinal bleeding, ascites, and edema in the central nervous system[60].
Due to the great impact caused by dengue in 1994, the Brazilian Ministry of Health prepared the Plan for Eradication of Ae. aegypti in 1996. A year later, it began the implementation of resources in municipalities convened the plan. This plan provided for an integrated action involving entomology, surveillance of ports and airports, sanitation, health information, epidemiological surveillance, and laboratories. However, due to the lack of distribution of resources, the actions were reduced practically to chemical control[57].
Since then, there has been an expansion of the areas inhabited by the vector and maintenance of high rates of infestation. Until 2000, dengue cases in the state were increasing, but still with incidences below
DENGUE DETERMINANTS
Given the conditions present in Brazil, understanding the epidemiology of dengue requires knowledge beyond those related to viral and vectorial aspects. It is also essential to evaluate the social, behavioral, economic, and environmental determinants[62].
Dengue is considered a neglected tropical disease, and similar to other diseases, it is influenced by social conditioning. Identifying social inequalities in the health-disease process and understanding the processes that produce them are essential conditions for finding ways to address the disease[63]. Analyzing studies carried out in different states in Brazil, it can be seen that dengue behaves according to the reality of each location, being influenced by socioeconomic factors.
In Natal, the capital of the state of Rio Grande do Norte, the incidence of dengue during a five-year historical series was not shown to be correlated with socioeconomic indicators, such as precariousness in sanitary structure or illiteracy rate[64]. This diverges from what was found in an epidemic in the state of Rio de Janeiro, where localities characterized by basic sanitation deficit were those with the highest incidence coefficients, presenting a strong correlation between these two variables[65].
The divergence of results among studies that seek to relate the incidence of the disease and the socioeconomic conditions of a population is common. Some authors bring a proportionally inverse relationship between these two variables, while others point out a directly proportional relationship[66]. Each place has a history resulting from simultaneous social and political processes and can thus show the particularity of the transmission of the disease in which production and reproduction occur[67].
The influence of climate variables leads to more homogeneous results. Climate variables have a statistically significant influence on the emergence of new cases in much of the country. Based on observations made in southern Brazil, Mendonça et al.[68] showed that the distribution of mosquitoes, the frequency of their bites, and the extrinsic incubation period are affected by temperature, rainfall, and wind speed. Mild temperatures, high humidity, and the occurrence of rainfall provide the conditions for Ae. aegypti to reproduce, survive for longer, and be viable to transmit the disease[16].
Climate variables end up directly influencing mosquito infestation, but a high infestation does not always cause many cases of the disease, just as a low infestation does not prevent the emergence of epidemics. In a study conducted in the municipality of Araraquara (São Paulo), infestation levels increased with rainfall and were reflected in the incidence coefficients of the disease[69]. In Fortaleza, even with a relatively low infestation by Ae. aegypti, according to most of the infestation index surveys carried out, epidemics occurred, such as in 2011[70].
The findings on the determinants of dengue are still vague and controversial, requiring analysis and understanding of the reality of each study area, since the determinants of disease vary according to its population, environmental, social, and economic dynamics[64,66].
DENGUE AND ITS DISTRIBUTION IN SPACE AND TIME
The role of epidemiology is to analyze the distribution of diseases and their determinants in the population, in space and time, as well as to unveil the social inequalities that influence the health-disease process[71].
Over the years, the concept of space has been modified; its definition has ceased to be only the geographical delimitation of a given area and started to aggregate different contexts and social, environmental, economic, and political determinants that can affect health of a population. Thus, it can be considered a valuable tool that allows understanding how the health-disease process occurs in the field of collective health[72].
For epidemiological studies, spatial analysis allows the recognition of clusters of events in a given population, thus identifying areas considered to be at risk for the occurrence of a disease, as well as subsidizing the planning of control and prevention actions[72].
In Brazil, these analysis techniques began to be used in 1980 and, although with many limitations at the time, managed to validate the importance of the environment and social dynamics in determining diseases[73]. Studies such as these are increasingly being applied to dengue, as it is intriguing to have high incidences and successive epidemics of this disease in a country that has managed to eliminate or control the morbidity and mortality of many infectious diseases.
Through spatial analysis, it was identified that there is a high burden of dengue in urban communities in Brazil, indicating that factors associated with the economic gradient influence the transmission of the disease[74]. It is also known that the incidence of dengue in some places is related to the agglomeration of people (high population density), and even at low infestation, there was the maintenance of active transmission of the virus in periods without epidemics[72,75].
Thus, having space as a unit of analysis is of extreme relevance, given the possibility of recognizing prediction factors for disease and interpreting them considering the reality of the study area.
First used in 1966 by Wiener, the term time series was defined as “sequences of quantitative data relating to specific points in time and studied according to their distribution in time”. A time series analysis is composed of four components: trend, cycle, seasonality, and external variation (white noise)[76,77].
The trend is explained as the prolonged movement in an ordered series, which can assume an increasing, decreasing, or stationary behavior. A study conducted by Böhm et al.[78] revealed that dengue showed a stationary trend throughout Brazil between the years 2002 and 2012, even though there was an increase in cases in some federal units, such as Tocantins and Alagoas. However, even if the incidence coefficient has behaved stably during the 11 years of analysis, the disease continues to burden the population. The cycles or cyclical variations are fluctuations in the values of the variable with a duration of more than one year, and which are repeated with a certain periodicity. Some authors do not work with this component, as it usually requires a time series of decades, and very old data can often be far from the real value.
Seasonality can be understood as repetitions of an event in an organized manner within a year, i.e., a seasonal phenomenon is defined as one that occurs regularly at fixed periods during an observed year. In Brazil, dengue is of seasonal occurrence, with high incidence in the first five months of the year, which is the hottest and most humid period[77,79]. The external variation or white noise is a component that coexists with the others when analyzing a historical series and can be visualized in the form of roughness in the graph lines[77].
CONCLUSION
Dengue has a historical series in Brazil of over 30 years and, between epidemics and non-epidemic years, has established itself as an endemic disease. This is due to the fact that there are areas in Brazil that offer favorable environmental conditions, such as high temperatures and high air humidity. There are also municipalities with low socioeconomic conditions and frequent water crises. Moreover, it is a tourist hub with an intense flow of visitors, keeping the doors open for the entry and circulation of diseases.
DECLARATIONS
Authors’ contributionsMade substantial contributions to the conception of the study: Martins ABS, Alencar CH
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Leandro AS, Lopes RD, Martins CA, et al. The adoption of the One Health approach to improve surveillance of venomous animal injury, vector-borne and zoonotic diseases in Foz do Iguaçu, Brazil. PLoS Negl Trop Dis 2021;15:e0009109.
2. Mavian C, Dulcey M, Munoz O, Salemi M, Vittor AY, Capua I. Islands as hotspots for emerging mosquito-borne viruses: a one-health perspective. Viruses 2018;11:11.
3. Gwee SXW, St John AL, Gray GC, Pang J. Animals as potential reservoirs for dengue transmission: a systematic review. One Health 2021;12:100216.
4. Mubemba B, Mburu MM, Changula K, et al. Current knowledge of vector-borne zoonotic pathogens in Zambia: a clarion call to scaling-up “One Health” research in the wake of emerging and re-emerging infectious diseases. PLoS Negl Trop Dis 2022;16:e0010193.
5. . World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. Genebra: World Health Organization; 2009. p. 147.
6. Reich NG, Shrestha S, King AA, et al. Interactions between serotypes of dengue highlight epidemiological impact of cross-immunity. J R Soc Interface 2013;10:20130414.
7. Rizzi C, Rizzi RL, Pramiu VP, Hoffmann E, Codeço C. Considerations about dengue fever and variable of importance to infestation by Aedes aegypti. Rev Bras Geo Med Saúde 2017;13:24-40.
8. Weaver SC, Vasilakis N. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect Genet Evol 2009;9:523-40.
9. Kuhn RJ, Zhang W, Rossmann MG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002;108:717-25.
10. Cruz-Oliveira C, Freire JM, Conceição TM, Higa LM, Castanho MA, Da Poian AT. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol Rev 2015;39:155-70.
11. Rodenhuis-Zybert IA, Wilschut J, Smit JM. Dengue virus life cycle: viral and host factors modulating infectivity. Cell Mol Life Sci 2010;67:2773-86.
12. Centers for Disease Control. Biology and Control of Aedes aegypti. Available from: https://stacks.cdc.gov/view/cdc/7670/cdc_7670_DS1.pdf [Last accessed on 22 Mar 2022].
13. Teixeira MDG, Barreto ML. Porque Devemos, de Novo, Erradicar o Aedes Aegypti. Ciênc saúde coletiva 1996;1:122-36.
14. Ibáñez-Bernal S, Briseño B, Mutebi JP, et al. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Vet Entomol 1997;11:305-9.
15. Teixeira MG, Barreto ML, Guerra Z. Epidemiology and preventive measures of Dengue. Inf Epidemiol SUS 1999;8:5-33.
16. Consoli RAGB, Oliveira RL. Main mosquitoes of sanitary importance in Brazil. Rio de Janeiro: Fiocruz; 1994.
17. Centers for Disease Control and Prevention. Vigilância e controle do Aedes aegypti e Aedes albopictus nos Estados Unidos United States: Centers for Disease Control and Prevention. Available from: https://portugues.cdc.gov/zika/vector/vector-control.html [Last accessed on 22 Mar 2022].
18. Gil LHS, Katsuragawa TH, Lima AAD, Tada MS, Ozaki LS, Julião GR. Rudimentary cesspits as breeding sites for Aedes aegypti in urban areas of Northern Brazil. Rev Pan-Amaz Saude 2015;6:73-80.
19. Martins VE, Alencar CH, Facó PE, et al. [Spatial distribution and breeding site characteristics of Aedes albopictus and Aedes aegypti in Fortaleza, State of Ceará]. Rev Soc Bras Med Trop 2010;43:73-7.
20. Martins VE, Alencar CH, Kamimura MT, et al. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceará, Brazil. PLoS One 2012;7:e41386.
21. Serufo JC, de Oca HM, Tavares VA, et al. Isolation of dengue virus type 1 from larvae of Aedes albopictus in Campos Altos city, State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 1993;88:503-4.
22. Sim S, Ramirez JL, Dimopoulos G. Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior. PLoS Pathog 2012;8:e1002631.
24. Zara AL, Santos SM, Fernandes-Oliveira ES, Carvalho RG, Coelho GE. [Aedes aegypti control strategies: a review]. Epidemiol Serv Saude 2016;25:391-404.
25. Abad-Franch F, Zamora-Perea E, Ferraz G, Padilla-Torres SD, Luz SL. Mosquito-disseminated pyriproxyfen yields high breeding-site coverage and boosts juvenile mosquito mortality at the neighborhood scale. PLoS Negl Trop Dis 2015;9:e0003702.
26. Braga IA, Valle D. Aedes aegypti: insecticides, mechanisms of action and resistance. Epidemiol Serv Saúde 2007;16:279-93.
27. Brasil. Epidemiological surveillance guide/Secretaria de Vigilância em Saúde/Departamento de Vigilância Epidemiológica. 7 ed. Brasilia: Ministério da Saúde do Brasil; 2009. Available from: https://www.saude.mg.gov.br/images/documentos/Guia%20de%20Vigilancia%207%20ed.pdf [Last accessed on 22 Mar 2022].
28. Gonçalves RP, Lima ECD, Lima JWDO, Silva MGCD, Caprara A. Recent contributions about the Brazilian population’s knowledge, attitudes and practices regarding dengue. Saúde Soc 2015;24:578-93.
29. Caprara A, Lima JW, Peixoto AC, et al. Entomological impact and social participation in dengue control: a cluster randomized trial in Fortaleza, Brazil. Trans R Soc Trop Med Hyg 2015;109:99-105.
30. Pustiglione M. Occupational Medicine and emerging, reemerging and neglected diseases: the conduct in the case of dengue, Chikungunya and Zika virus. Rev Bras Med Trab 2016;14:1-12.
31. Dengue diagnosis and clinical management adult and children. 5 ed. Brasília: Ministério da Saúde do Brasil. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/publicacoes-svs/dengue/dengue-manejo-adulto-crianca-5d-1.pdf [Last accessed on 22 Mar 2022].
32. Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res 2002;33:330-42.
33. Saito CK, Machado SCP, Medina WSG, Paoliello-Paschoalato AB. Serology and clinical evaluation: correlation in the diagnosis of dengue. Cuid Art Enferm 2017;11:72-7.
35. Lupi O, Carneiro CG, Coelho ICB. Mucocutaneous manifestations of dengue. An Bras Dermatol 2007;82:291-305.
36. Halstead SB. The Alexander D. Langmuir Lecture. The pathogenesis of dengue. Molecular epidemiology in infectious disease. Am J Epidemiol 1981;114:632-48.
38. Fragoso YD, Gomes S, Brooks JB, et al. Guillain-Barré syndrome and dengue fever: report on ten new cases in Brazil. Arq Neuropsiquiatr 2016;74:1039-40.
39. Simon O, Billot S, Guyon D, et al. Early Guillain-Barré Syndrome associated with acute dengue fever. J Clin Virol 2016;77:29-31.
40. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013;496:504-7.
41. Duong V, Lambrechts L, Paul RE, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A 2015;112:14688-93.
42. Rigau-pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vance Vorndam A. Dengue and dengue haemorrhagic fever. Lancet 1998;352:971-7.
43. Messina JP, Brady OJ, Scott TW, et al. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 2014;22:138-46.
44. Dick O, San Martín JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012;87:584-93.
45. Guo C, Zhou Z, Wen Z, et al. Global epidemiology of dengue outbreaks in 1990-2015: a systematic review and meta-analysis. Front Cell Infect Microbiol 2017;7:317.
46. Fogaça TK, Mendonça FdA. Dengue fever in the Americas: spatial distribution and viral circulation. Hygeia 2017;13:175-88.
47. Perez F, Llau A, Gutierrez G, et al. The decline of dengue in the Americas in 2017: discussion of multiple hypotheses. Trop Med Int Health 2019;24:442-53.
48. Fares RC, Souza KP, Añez G, Rios M. Epidemiological scenario of dengue in Brazil. Biomed Res Int 2015;2015:321873.
49. Nogueira RM, Schatzmayr HG, de Filippis AM, et al. Dengue virus type 3, Brazil, 2002. Emerg Infect Dis 2005;11:1376-81.
50. Temporao JG, Penna GO, Carmo EH, et al. Dengue virus serotype 4, Roraima State, Brazil. Emerg Infect Dis 2011;17:938-40.
51. Romano CM, de Matos AM, Araújo ES, et al. Characterization of Dengue virus type 2: new insights on the 2010 Brazilian epidemic. PLoS One 2010;5:e11811.
52. . Brasil. Epidemiological Bulletin. Monitoring of dengue and chikungunya fever cases until the 53th Epidemiological Week, 2014. Brasilia: Ministério da Saúde, Secretaria de Vigilância em Saúde; 2015. (in Portuguese)
53. . Brasil. Epidemiological Bulletin. Monitoring of dengue and chikungunya fever cases until the 52th Epidemiological Week, 2015. Brasilia: Ministério da Saúde, Secretaria de Vigilância em Saúde; 2016. (in Portuguese)
54. . Brasil. Epidemiological Bulletin. Monitoring of dengue and chikungunya fever cases until the 52th Epidemiological Week, 2016. Brasilia: Ministério da Saúde, Secretaria de Vigilância em Saúde; 2017. (in Portuguese)
55. . Brasil. Epidemiological Bulletin. Monitoring of dengue and chikungunya fever cases until the 52th Epidemiological Week, 2017. Brasília: Ministério da Saúde, Secretaria de Vigilância em Saúde; 2018. (in Portuguese)
56. . Brasil. Epidemiological Bulletin. Monitoring of dengue cases, chikungunya fever and acute illness by Zika virus until the 52th Epidemiological Week 52, 2018. Brasília: Ministério da Saúde, Secretaria de Vigilância em Saúde; 2019. (in Portuguese)
57. Lima EP, Goulart MOF, Albuquerque MR, Victor FM, Pinto NB. Time series analysis of incidence of dengue and Aedes aegypti in Ceará. Brazilian Journal in Health Promotion 2013;26:336-43.
58. Cunha R V, Miagostovich M P, Petrola Z. Retrospective study on dengue in Fortaleza, state of Ceará, Brazil. Mem Inst Oswaldo Cruz 1998;93:155-9.
59. Cavalcanti LPG, Almeida Barreto FK, Oliveira RMAB, et al. Thirty years of dengue in Ceará: history, contributions to science and challenges in the current scenario with triple arbovirus circulation. J Health Biol Sci 2017;6:65-82.
60. Vasconcelos PF, de Menezes DB, Melo LP, et al. A large epidemic of dengue fever with dengue hemorrhagic cases in Ceará State, Brazil, 1994. Rev Inst Med Trop Sao Paulo 1995;37:253-5.
61. Vasconcelos PF, Lima JW, da Rosa AP, et al. Dengue epidemic is a Northeastern Brazil: randon epidemiological serum survey. Rev Saúde Pública 1998;32:447-54.
62. MacCormack-Gelles B, Neto ASL, Sousa GS, et al. Epidemiological characteristics and determinants of dengue transmission during epidemic and non-epidemic years in Fortaleza, Brazil: 2011-2015. PLoS Negl Trop Dis 2018;12:e0006990.
63. Barata RB. How and why social inequalities are bad for your health. 1 ed. Rio de Janeiro: Editora Fiocruz; 2009.
64. Barbosa IR, da Silva LP. Influence of social and environmental determinants on the spatial distribution of dengue in the municipality of Natal-RN. Rev Ciênc Plural 2015;1:62-75.
65. Teixeira TR, Medronho Rde A. [Socio-demographic factors and the dengue fever epidemic in 2002 in the State of Rio de Janeiro, Brazil]. Cad Saude Publica 2008;24:2160-70.
66. Flauzino RF, Souza-Santos R, Oliveira RM. [Dengue, geoprocessing, and socioeconomic and environmental indicators: a review]. Rev Panam Salud Publica 2009;25:456-61.
67. Sabroza PC, Toledo Ld, Osanai CH. .
68. Mendonça FA, Souza AV, Dutra DA. Public health, urbanization and dengue’s fever in Brazil. Soc Nat 2009;21:257-69.
69. Ferreira AC, Chiaravalloti Neto F, Mondini A. Dengue in Araraquara, state of São Paulo: epidemiology, climate and Aedes aegypti infestation. Rev Saude Publica 2018;52:18.
70. Oliveira RMAB, Araújo FMC, Cavalcanti LPG. Entomological and epidemiological aspects of dengue epidemics in Fortaleza, Ceará, Brazil, 2001-2012. Epidemiol Serv Saude 2018;27:e201704414.
71. Hino P, Villa TC, Sassaki CM, Nogueira Jde A, dos Santos CB. Geoprocessing in health area. Rev Lat Am Enfermagem 2006;14:939-43.
72. Skalinski LM, Costa MDCN, Teixeira MDGL. Contributions of spatial analysis to the comprehension of dynamics of dengue transmission: integrative review. J Health Biol Sci 2018;7:53-63.
73. Carvalho MS, Souza-Santos R. [Analysis of spatial data in public health: methods, problems, and perspectives]. Cad Saude Publica 2005;21:361-78.
74. Kikuti M, Cunha GM, Paploski IA, et al. Spatial distribution of dengue in a brazilian urban slum setting: role of socioeconomic gradient in disease risk. PLoS Negl Trop Dis 2015;9:e0003937.
75. Almeida AS, Medronho Rde A, Valencia LI. Spatial analysis of dengue and the socioeconomic context of the city of Rio de Janeiro (Southeastern Brazil). Rev Saude Publica 2009;43:666-73.
76. Latorre MDRDDO, Cardoso MRA. Time series analysis in epidemiology: an introduction to methodological aspects. Rev bras epidemiol 2001;4:145-52.
77. Antunes JLF, Cardoso MRA. Using time series analysis in epidemiological studies. Epidemiol Serv Saúde 2015;24:565-76.
78. Böhm AW, Costa CD, Neves RG, Flores TR, Nunes BP. Dengue incidence trend in Brazil, 2002-2012. Epidemiol Serv Saude 2016;25:725-33.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Martins ABS, Alencar CH. Ecoepidemiology of dengue in Brazil: from the virus to the environment. One Health Implement Res 2022;2:1-14. http://dx.doi.org/10.20517/ohir.2021.10
AMA Style
Martins ABS, Alencar CH. Ecoepidemiology of dengue in Brazil: from the virus to the environment. One Health & Implementation Research. 2022; 2(1): 1-14. http://dx.doi.org/10.20517/ohir.2021.10
Chicago/Turabian Style
Martins, Ana Beatriz Souza, Carlos Henrique Alencar. 2022. "Ecoepidemiology of dengue in Brazil: from the virus to the environment" One Health & Implementation Research. 2, no.1: 1-14. http://dx.doi.org/10.20517/ohir.2021.10
ACS Style
Martins, ABS.; Alencar CH. Ecoepidemiology of dengue in Brazil: from the virus to the environment. One. Health Implement. Res. 2022, 2, 1-14. http://dx.doi.org/10.20517/ohir.2021.10
About This Article
Copyright
Data & Comments
Data
 Cite This Article 94 clicks
Cite This Article 94 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.